Thermodynamics
1. Introduction
- Thermodynamics is that branch of physics which is concerned with transformation of heat into mechanical work.
- It deals with the concepts of heat, temperature and interconversion of heat into other forms of energy i.e., electrical, mechnical, chemical magnetic etc.
- Thermodynamics does not take any account of atomic or molecular constitution of matter and it deals with the bulk systems.
- State of any thermodynamic system can be described in terms of certain know macroscopic variables known as thermodynamic variables.
- Thermodynamic variables determine the thermodynamic behaviour of a system . Quantities like pressure(P), volume(V), and temperature(T) are thermodynamic variables. Some other thermodynamic variables are entropy, internal energy etc. described in terms of P, V and T
- A thermodynamic system is said to be in thermal equilibrium if all parts of it are at same temperature.
- Thus two systems are said to be in thermal equilibrium if they are at same temperature.
2. Concept of Heat
- Heat may be defined as energy in transit.
- Word heat is used only if there is a transfer of energy from one thermodynamic system to the another.
- When two systems at different temperatures are kept in contect with each other then after some time temperatures of both the syatems become equal and this phenomenon can be described by saying that energy has flown from one system to another.
- This flow of energy from one system to another on account of temperature difference is called heat transfer.
- Flow of heat is a non-mechanical mode of energy transfer.
- Heat flow depends not only on initial and find states but also on path it's.
3. P-V Indicator Digram
- Only two thermodynamic variables are sufficient to describe a system because third vaiable can be calculated from equation of state of the system.
- P-V Indicator Digram is just a graph between pressure and volume of a system undergoing an operation.
- When a system undergoes an expansion from state A (P1 V1) to a state B (P2V2) its indicator digram is shown as follows.
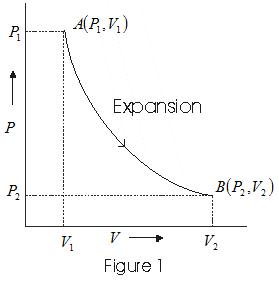
- In case of compression system at state A(P1 V1) goes to a state B(P2V2) its indicator digram is as follows.
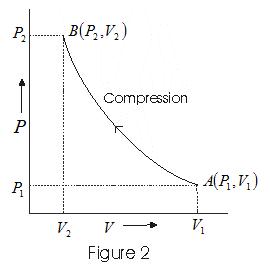
- Intermediate states of system are represented by points on the curve.
- The pressure volume curve for a fixed temperature is called isotherm.
4. Work in volume changes
- Consider a cylinder filled with gas and equiped with a movable piston as shown in fig below
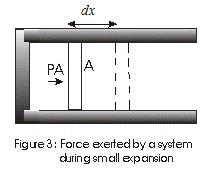
fig - Force exerted by a system during small expansion.
Suppose,
A - Cross Sectional area of cylinder
P - Pressure exerted by piston at the piston face.
PA - Force exerted by the system. - If piston moves out by a distance dx then work done by this force is dW given by
dW = PAdx
= PdV (1)
since V = Adx and dV is change in volume of the system. - In a finite volume change from V1 to V2
W=∫PdV (2)
where limits of integration goes from V1 to V2
Graphically this relationship is shown below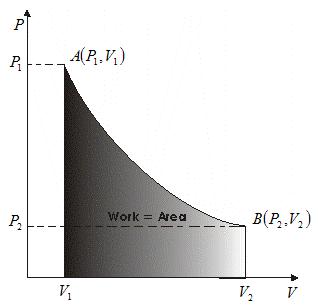
- Thus eqn (2) can be interpreted graphically as area under the curve between limits V1and V2.
- If pressure remains constant while the volume changes, then work is
W = P(V2-V1) (3) - Work done not only depends on initial and final states but also on the intermediate states i.e., on the path.
5. Internal Energy and first law of thermodynamics
- Internal energy can be described as the sum of kinetic and potantial energies of individual movecules in the material.
- But in thermodynamics one should keep in mind that U is simply a macrosopic variable of the system.
- U is thermodynamic state variable and its value depends only on the given state of the system and not on path taken to arrive the state.
- Transfer of heat and performance of work are two mean of adding or subtracting energy from a system.
- On transfer of energy, system is said to have undergone a change in internal energy.
- Thus the sum of heat put into the system plus work done on the system equals increase in internal energy of the system for any process.
if, U1 is internal energy of state 1 and U2 is internal energy of state 2 than change in internal energy would be
ΔU=U2 - U1 - If W is the work done by the system on its surroundings then -W would be the work done on the system by the surroundings .
- If Q is the heat put into the system then,
Q+(-W)=ΔU
usually written as
Q=ΔU+W (4) - Equation (4) is then know as first law of thermodynamics and it can be applied when
Q, W and U are expressed in same units.
Some Imp stuff (1) Q is positive when heat is given to the system and Q is negative when heat is taken from the system
(2) W is positive when system expands and does work on surroundings - Hence we may say that when a certain amount of heat Q is given to the system then some part of it is used in increasing internal energy ΔU of the system while remaining part leaves the system in form of work done by the system on its surroundings.
- From equation 4 we see that first law of thermodynamics is a statement of conservation of energy stated as
' The energy put into the system equals the sum of the work done by the system and the change in internal energy of the system' - If the system undergoes any process in which ΔU=0 i.e., charge in internal energy is zero then from (4)
Q = W
that is heat supplied to the system is used up enterely in doing work on the surroundings.
6. Specific heat capacity of an ideal gas
- We have defined specific heat capacity and molar specific heat capacity earlier in the previous chapter.
- There are two specific heats of ideal gases.
(i) Specific heat capacity at constant volume
(ii) Specific heat capacity at constant pressure
Cp and Cv are molar specific heat capacities of ideal gas at constant pressure and volume respectively for Cp and Cv of ideal gas there is a simple relation.
Cp-Cv = R (7)
where R- universal gascontant - This relation can be proved as follows.
from first law of thermaodynamics for 1 mole of gas we have
ΔQ =Δ U+PΔV (8) - If heat is absorbed at constant volume then ΔV = 0 and
CV=(ΔQ/ΔT)V=(ΔU/ΔT)V (9)
If Q in absorbed at constant pressure than
CP=(ΔQ/ΔT)P=(ΔU/ΔT)P+P(ΔV/ΔT)P
now ideal gas equation for 1 mole of gas is
PV = RT
= P(ΔV/ΔT) = R (10)
from (9) and (10)
CP - CV=(ΔU/ΔT)P-(ΔU/ΔT)V+P(ΔV/ΔT)P - Since internal energy U of ideal gas depands only on temperature so subscripts P and V have no meaning.
=> CP - CV = R
which is the desired relation
7. Thermodynamic Processes
(a) Quasi static Processes
:- In Quasi static process deviation of system from it's thermodynamic equilibrium is infinitesimally small.
- All the states through which system passed during a quasi static process may be regarded as equilibrium states.
- Consider a system in which gas is contained in a cylinder fitted with a movable piston then if the piston is pushed in a infinitely slow rate, the system will be in quiscent all the time and the process can be considered as quasi-static process.
- Vanishingly slowness of the process is an essential feature of quasi-static process.
- During quasi-static process system at every moment is infinitesimally near the state of thermodynamic equilibrium.
- Quasi static process is an idealized concept and its conditions can never be rigoursly satisfied in practice.
(b) Isothermal Process
:- In isothermal process temperature of the system remains constant throughout the process.
- For an iso-thermal process equation connecting P, V and T gives.
PV = constant
i.e., pressure of given mass of gas varies inversly with its volume this is nothing but the Boyle's law. - In iso thermal process there is no change in temperature, since internal energy for an ideal gas depends only on temperature hence in iso thermal process there is no change in internal energy.
Thus,
ΔU=0
therefore, ΔQ =ΔW - Thus during iso thermal process
Heat added (or substacted) from the system = wok done by (or on) the system
(c) Adiabatic Process
:- Process in which no heat enters or leaves a system is called an adiabatic process
- For every adiabatic process Q=0
- Prevention of heat flow can be acomplished by surrounding system with a thick layer of heat insulating material like cork, asbestos etc.
- Flow of heat requires finite time so if a process is perfomed very quickly then process will be pratically adiabatic.
- On applying first law to adiabatic process we get
ΔU=U2 - U1= - ΔW (adiabatic process) - In adiabatic process change in internal energy of a system is equal in magnitade to the work by the system.
- If work is done on the system contracts i.e. ΔW is nagative then.
ΔU = ΔW
and internal energy of system increases by an amount equal to the work done on it and temperature of system increases. - If work is done by the system i.e., ΔW is negative
ΔU = -Δ W
here internal energy of systems decreases resulting a drop in temperature.
(d) Isochoric process v:
- In an isochoric process volume of the system remain uncharged throughout i.e. ΔV = O.
- When volume does not change no work is done ; ΔW = 0 and therefore from first law
U2 - U1 = ΔU =ΔQ
- All the heat given to the system has been used to increase the intenal energy of the system.
(e) Isobaric Process
:
- A process taking place at constant pressure is called isobaric process.
- From equation (3) we see that work done in isobaric process is
W = P(V2 - V1) nR (T2-T1)
where pressure is kept constant.
- Here in this process the amount of heat given to the system is partly used in increasing temperature and partly used in doing work.
8. Work done in Isothermal process
- In an isothermal process temperature remains constant.
- Consider pressure and volume of ideal gas changes from (P1, V1) to (P2, V2) then, from first law of thermodynamics
ΔW = PΔV
Now taking ΔV aproaching zero i.e. ΔV and suming ΔW over entire process we get total work done by gas so we have
W = ∫PdV
where limits of integration goes from V1 to V2
as PV = nRT we have P = nRT / V
W = ∫(nRT/V)dV
where limits of integration goes from V1 to V2
on integrating we get,
W=nRT ln(V2/V1) (3)
Where n is number of moles in sample of gas taken.
9. Work done in an Adiabatic process
- For an adiabatic process of ideal gas equation we have
PVγ = K (Constant) (14)
Where γ is the ratio of specific heat (ordinary or molar) at constant pressure and at constant voluume
γ = Cp/Cv
- Suppose in an adiabatic process pressure and volume of a sample of gas changs from (P1, V1) to (P2, V2) then we have
P1(V1)γ=P2(V2)γ=K
Thus, P = K/Vγ
- Work done by gas in this process is
W = ∫PdV
where limits of integration goes from V1 to V2
Putting for P=K/Vγ, and integrating we get,
W = (P1V1-P2V2)/(γ-1) (16)
- In and adiabatic process if W>0 i.e., work is done by the gas then T2< T1
- If work is done on the gas (W<0) then T2 > T1 i.e., temperature of gas rises.
10. Heat Engine and efficiency
- Any device which convents heat continously into mechenical work is called a heat engine.
- For any heat engine there are three essential requirements.
(i) SOURCE : A hot body at fixed temperature T1 from which heat engine can draw heat
(ii) Sink : A cold body, at a fixed lower temprature T2, to which any amount of heat can be rijectd.
(iii) WOEKING SUBTANCE : The material, which on being supplied with heat will do mechanical work.
- In heat engine, working substances, could be gas in cylinder with a moving piston.
- In heat engine working substance takes heat from the sorce, convents a part of it into mechanical work, gives out rest to the sink and returns to the initial state. This series of operations constitutes a cycle.
- This cycle is represented in fig below
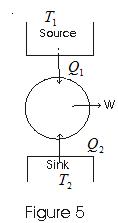
- Work from heat engine can be continously obtained by performing same cycle again and again.
- Consider,
Q1 - heat absorbed by working substance from sorce
Q2 - heat rejected to the since
W - net amount of work done by working substance
Q1-Q2 - net amount of heat absorbed by working substance.
ΔU = 0 since in the cycle Working Substance returns to its initial condition.
So on application of first law of thermodynamics
Q1-Q2 = W
- Thermal efficiency of heat engine
η= work output in energy units / Heat input in same energy units
= W / Q1 = (Q1-Q2 )/ Q1
Or, η = 1-(Q2/Q1) (17)
from this equation it is clear that
Q = 1 for Q2=0
and there would be 100% conversion of heat absorbed into work but such ideal engines are not possible in practice.
11. Principle of a Refrigerator
- Refrigerators works in reverse direction of heat engines.
- In refrigerators working substance extracts heat Q2 from sink at lower temperature T2
- Some external work is performed by the compressor of refrigerator and then heat Q1 is rejected to the
source, to the radiator of the refrigerator.
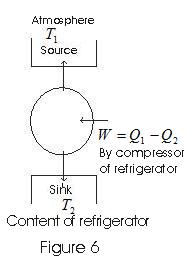
Coefficent of performance :
β= Amount of heat absorbed from the cold reservoir / work done in running the mechinery
Q2 - heat absorbed from cold reservoir.
Q1 - heat rejected to hot reservoir during one complete cycle
W = (Q1-Q2 ) is the work done in running the machinery
thus,
β= Q2/W =Q2/(Q1-Q2) (18)
- Like heat engines refrigerators can not work without some external work done on the system. Hence coefficent of performance can not be infinite.
12. Second law of thermodynamics
- First law of thermodynamics states the equivalance of heat and energy.
- It does not state anything about the limitation in the conversion of heat into work or about the condition necessary for such conversion.
- Second law of thermodynamics is generalization of certain experience and observation and is concerned with tine direction in which energy flow takes place.
- This law can be stated in number of ways. Although differently said, they are essentially equvalent.
(i)Kelvin Plank Statment :
"It is impossible to construct a device which, operating in a cycle, has a sole effect of extracting heat from a reservoir and performing an equivalent amount of work".
(ii)Clasius Statement :
"It is impossible for a self acting machine, unaided by enternal agency, to transfer heat from a colder body to a hotter body".
- It can ne proved that these two statements of second law are completely equivalent and voilation of Kelvin Plank statement leads to voilation of Clasius statement and vice-versa.
13. Reversibility and irreversibility
- Reversible process is the one which can be retraced in opposite order by changing external conditions slightly.
- Those processes which can not be retraced in opposite order by reversing the controling factors are known as irreversible process.
- It is a consequence of second law that all the natural processes are irreversible process.
- Conditions for reversibility of a process are
(i) Process is performed quasi-statically
(ii) it is not accompained by any dissipative effects.
- It is impossible to satisfy these two conditions perfectly, thus requessible process is purely an ideal abstraction.
14. Carnot's Heat Engine
- According to second law of thermodynamics, no heat engine can have 100% efficiency
- Carnot’s heat engine is an idealized heat engine that has maximum possible efficiency consistent with the second law.
- Cycle through which working substance passed in Carnot’s engine is known as Carnot’s Cycle.
- Carnot's engine works between two temperatures
T1 - temperature of hot reservoir
T2 - temperature of cold reservoir
- In a Complete Carnot's Cycle system is taken from temperature T1 to T2 and then back from
temerature T2 to T1.
- We have taken ideal gas as the working substance of cornot engine.
- Fig below is an indicator digram for Cornot Cycle of an ideal gas
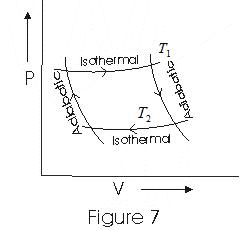
(i) In step b→c iso thermal esepansion of gas taken place and thermodynamic variables of gas changes from (P1, V1,T1) to (P2,V2,T1)
- If Q1 is the amount of heat absorbed by working substance from the source and W1 the work done by the gas then from eqn (13)
Q1 = W1 = nRT1 ln (V2/V1) (19)
as process is iso thermal.
(ii) Step c→d is an adiabatic expension of gas from (P2, V2, T1) to (P3,V3,T2). Work done by gas in adiabatic esepansion is given by eqn (16)
W2 = nR (T1-T2)/(γ-1) (20)
(iii)Step d→a is iso-thermal compression of gas from (P3,V3,T2) to (P4,V4,T2). Heat Q2 would be released by the gas to the at temperature T2
- Work done on the gas by the environment is
W3 = Q2
= nRT2ln(V3/ V4) (21)
(iv)Step a→b is adiabatic compression of gas from (P4, V4, T2) to (P1, V1, T1)
- Work done on the gas is
W4 =nR (T1-T2)/(γ-1) (22)
- Now total work done in one complete cycle is
W = W1 + W2 - W3 - W4
= nRT1ln(V2/V1)-nRT2ln(V3/V4) (23)
as W2 = W4
- Efficency of carnot engine
η=W/Q1 = 1-(Q2/Q1)
= 1-(T2/T1)ln(V3/V4)/ln(V2/V1) (24)
or η= 1-[T2ln(V3/V4)/T1ln(V2/V1)] (25)
Since points b and c lie on same iso thermal
⇒ P1V1=P2V2 (26)
also points c and d lie on same adiabatic
⇒ P2(V2)γ=P3(V3)γ (27)
also points d and a lie on same iso thermal and points a and b on sum adiabatic thus,
P3V3=P4V4 (28)
P4(V4)γ=P2(V1)γ (29)
multiplying all the above four eqns me get
V3/4 = V2/V1 (30)
Putting this in equation (25) we get
η= 1-(T2/T1) (31)
From above eqn we can draw following conclusions that efficency of Carnot engine is
(i) independent of the nature of working substance
(ii) depend on temperature of source and sink
15. Carnot Theorem
- Carnot Engine is a reversible engine.
- Carnot’s theorem consists of two parts
(i) no engine working between two given temperatures can be more efficent than a reversible Carnot engine working between same source and sink.
(ii) all reversible engines working between same source and sink (same limits or temperature) have the same efficiency irrespective the working substance.
Solved Examples
Question-1. What is true of Isothermal Process
a, ΔT >0
b, ΔU=0
c ΔQ=ΔW
d PV=constants
Solution-1:
In an Isothermal Process
Temperature remains constant ΔT =0
Since Internal energy depends on the temperature
ΔU=0
From first law of Thermodynamics
ΔU=ΔQ-ΔW
Since ΔU=0
ΔQ=ΔW
Also PV=nRT
As T is constant
PV= constant
Question-.2 Two absolute scales A and B have triple points of water defined as 200A and 350A. what is the relation between TA and TB
Solution-2
Given that on absolute scale
Triple point of water on scale A = 200 A
Triple point of water on scale B = 350 B
Also, triple point of water on Kelvin scale = 273.16 K
Now temperature on scale A and on scale B is equivalent to 273.16 K on absolute temperature scale.
Thus, value of one degree on absolute scale A = (273.16/200) K
Or,
Value of temperature TA on absolute scale A = (273.16XTA)/200
Similarly value of temperature TB on absolute scale B = (273.16XTB)/350
Since TA and TB represent the same temperature
273.16×TA/200 = 273.16×TB/350
Or, TA = 200TB/350 = 4TB/ 7
Question 3:A gas is contained in a cylinder with a moveable piston on which a heavy block is placed. Suppose the region outside the chamber is evacuated and the total mass of the block and the movable piston is 102 kg. When 2140 J of heat flows into the gas, the internal energy of the gas increases by 1580 J. What is the distance s through which the piston rises?
Solution:3
Total heat supplied =Workdone + Change in internal energy
So work done=2140-1580=560 J
Let s be the distance moved then
the workdone is given by =Fs
Fs=560
s=560/F
=560/102*10
s=.54 m
Question-4: At 27°C,two moles of an ideal monoatomic gas occupy a volume V.The gas is adiabatically expanded to a volume 2V.
Calculate the ratio of final pressure to the intial pressure
Calculate the final temperature
Change in internal energy
Calculate the molar specific heat capacity of the process
Solution-4
Given
n=2 T=27°C=300 K ,V1=V,V2=2V
Now PVy=constant
P1V1y=P2V2y
P2/P2=(V1/V2)y
=.55/3
ALso
T1V1y-1=T2V2y-1
or T2=300/25/3=189K
Change in internal energy=nCvΔT
For monoatomic gas Cv=3R/2
Substituting all the values
Change in internal energy==-2764J
As in adiabatic process ΔQ=0,molar specific heat capacity=0
Question-5
An ideal gas heat engine operates in Carnot cycle between 227°C and 127°C.It absorbs 6*102 cal of heat at the higher temperature.Calculate the amount of heat supplied to the engine from the source in each cycle
Solutions-5:
T1=227°C =500K
T2=127°C =400K
Efficiency of the carnot cycle is given by
=1-(T2/T1)=1/5
Now also efficency =Heat supplied from source/Heat absorbed at high temperature
so Heat supplied from source=6*102*(1/5)==1.2*102cal
U2 - U1 = ΔU =ΔQ
W = P(V2 - V1) nR (T2-T1)
where pressure is kept constant.
8. Work done in Isothermal process
- In an isothermal process temperature remains constant.
- Consider pressure and volume of ideal gas changes from (P1, V1) to (P2, V2) then, from first law of thermodynamics
ΔW = PΔV
Now taking ΔV aproaching zero i.e. ΔV and suming ΔW over entire process we get total work done by gas so we have
W = ∫PdV
where limits of integration goes from V1 to V2
as PV = nRT we have P = nRT / V
W = ∫(nRT/V)dV
where limits of integration goes from V1 to V2
on integrating we get,
W=nRT ln(V2/V1) (3)
Where n is number of moles in sample of gas taken.
9. Work done in an Adiabatic process
- For an adiabatic process of ideal gas equation we have
PVγ = K (Constant) (14)
Where γ is the ratio of specific heat (ordinary or molar) at constant pressure and at constant voluume
γ = Cp/Cv - Suppose in an adiabatic process pressure and volume of a sample of gas changs from (P1, V1) to (P2, V2) then we have
P1(V1)γ=P2(V2)γ=K
Thus, P = K/Vγ - Work done by gas in this process is
W = ∫PdV
where limits of integration goes from V1 to V2
Putting for P=K/Vγ, and integrating we get,
W = (P1V1-P2V2)/(γ-1) (16) - In and adiabatic process if W>0 i.e., work is done by the gas then T2< T1
- If work is done on the gas (W<0) then T2 > T1 i.e., temperature of gas rises.
10. Heat Engine and efficiency
- Any device which convents heat continously into mechenical work is called a heat engine.
- For any heat engine there are three essential requirements.
(i) SOURCE : A hot body at fixed temperature T1 from which heat engine can draw heat
(ii) Sink : A cold body, at a fixed lower temprature T2, to which any amount of heat can be rijectd.
(iii) WOEKING SUBTANCE : The material, which on being supplied with heat will do mechanical work. - In heat engine, working substances, could be gas in cylinder with a moving piston.
- In heat engine working substance takes heat from the sorce, convents a part of it into mechanical work, gives out rest to the sink and returns to the initial state. This series of operations constitutes a cycle.
- This cycle is represented in fig below

- Work from heat engine can be continously obtained by performing same cycle again and again.
- Consider,
Q1 - heat absorbed by working substance from sorce
Q2 - heat rejected to the since
W - net amount of work done by working substance
Q1-Q2 - net amount of heat absorbed by working substance.
ΔU = 0 since in the cycle Working Substance returns to its initial condition.
So on application of first law of thermodynamics
Q1-Q2 = W - Thermal efficiency of heat engine
η= work output in energy units / Heat input in same energy units
= W / Q1 = (Q1-Q2 )/ Q1
Or, η = 1-(Q2/Q1) (17)
from this equation it is clear that
Q = 1 for Q2=0
and there would be 100% conversion of heat absorbed into work but such ideal engines are not possible in practice.
11. Principle of a Refrigerator
- Refrigerators works in reverse direction of heat engines.
- In refrigerators working substance extracts heat Q2 from sink at lower temperature T2
- Some external work is performed by the compressor of refrigerator and then heat Q1 is rejected to the
source, to the radiator of the refrigerator.
Coefficent of performance :
β= Amount of heat absorbed from the cold reservoir / work done in running the mechinery
Q2 - heat absorbed from cold reservoir.
Q1 - heat rejected to hot reservoir during one complete cycle
W = (Q1-Q2 ) is the work done in running the machinery
thus,
β= Q2/W =Q2/(Q1-Q2) (18) - Like heat engines refrigerators can not work without some external work done on the system. Hence coefficent of performance can not be infinite.
12. Second law of thermodynamics
- First law of thermodynamics states the equivalance of heat and energy.
- It does not state anything about the limitation in the conversion of heat into work or about the condition necessary for such conversion.
- Second law of thermodynamics is generalization of certain experience and observation and is concerned with tine direction in which energy flow takes place.
- This law can be stated in number of ways. Although differently said, they are essentially equvalent.
(i)Kelvin Plank Statment :
"It is impossible to construct a device which, operating in a cycle, has a sole effect of extracting heat from a reservoir and performing an equivalent amount of work".
(ii)Clasius Statement :
"It is impossible for a self acting machine, unaided by enternal agency, to transfer heat from a colder body to a hotter body". - It can ne proved that these two statements of second law are completely equivalent and voilation of Kelvin Plank statement leads to voilation of Clasius statement and vice-versa.
13. Reversibility and irreversibility
- Reversible process is the one which can be retraced in opposite order by changing external conditions slightly.
- Those processes which can not be retraced in opposite order by reversing the controling factors are known as irreversible process.
- It is a consequence of second law that all the natural processes are irreversible process.
- Conditions for reversibility of a process are
(i) Process is performed quasi-statically
(ii) it is not accompained by any dissipative effects. - It is impossible to satisfy these two conditions perfectly, thus requessible process is purely an ideal abstraction.
14. Carnot's Heat Engine
- According to second law of thermodynamics, no heat engine can have 100% efficiency
- Carnot’s heat engine is an idealized heat engine that has maximum possible efficiency consistent with the second law.
- Cycle through which working substance passed in Carnot’s engine is known as Carnot’s Cycle.
- Carnot's engine works between two temperatures
T1 - temperature of hot reservoir
T2 - temperature of cold reservoir - In a Complete Carnot's Cycle system is taken from temperature T1 to T2 and then back from
temerature T2 to T1. - We have taken ideal gas as the working substance of cornot engine.
- Fig below is an indicator digram for Cornot Cycle of an ideal gas

(i) In step b→c iso thermal esepansion of gas taken place and thermodynamic variables of gas changes from (P1, V1,T1) to (P2,V2,T1) - If Q1 is the amount of heat absorbed by working substance from the source and W1 the work done by the gas then from eqn (13)
Q1 = W1 = nRT1 ln (V2/V1) (19)
as process is iso thermal.
(ii) Step c→d is an adiabatic expension of gas from (P2, V2, T1) to (P3,V3,T2). Work done by gas in adiabatic esepansion is given by eqn (16)
W2 = nR (T1-T2)/(γ-1) (20)
(iii)Step d→a is iso-thermal compression of gas from (P3,V3,T2) to (P4,V4,T2). Heat Q2 would be released by the gas to the at temperature T2 - Work done on the gas by the environment is
W3 = Q2
= nRT2ln(V3/ V4) (21)
(iv)Step a→b is adiabatic compression of gas from (P4, V4, T2) to (P1, V1, T1) - Work done on the gas is
W4 =nR (T1-T2)/(γ-1) (22) - Now total work done in one complete cycle is
W = W1 + W2 - W3 - W4
= nRT1ln(V2/V1)-nRT2ln(V3/V4) (23)
as W2 = W4 - Efficency of carnot engine
η=W/Q1 = 1-(Q2/Q1)
= 1-(T2/T1)ln(V3/V4)/ln(V2/V1) (24)
or η= 1-[T2ln(V3/V4)/T1ln(V2/V1)] (25)
Since points b and c lie on same iso thermal
⇒ P1V1=P2V2 (26)
also points c and d lie on same adiabatic
⇒ P2(V2)γ=P3(V3)γ (27)
also points d and a lie on same iso thermal and points a and b on sum adiabatic thus,
P3V3=P4V4 (28)
P4(V4)γ=P2(V1)γ (29)
multiplying all the above four eqns me get
V3/4 = V2/V1 (30)
Putting this in equation (25) we get
η= 1-(T2/T1) (31)
From above eqn we can draw following conclusions that efficency of Carnot engine is
(i) independent of the nature of working substance
(ii) depend on temperature of source and sink
15. Carnot Theorem
- Carnot Engine is a reversible engine.
- Carnot’s theorem consists of two parts
(i) no engine working between two given temperatures can be more efficent than a reversible Carnot engine working between same source and sink.
(ii) all reversible engines working between same source and sink (same limits or temperature) have the same efficiency irrespective the working substance.
a, ΔT >0
b, ΔU=0
c ΔQ=ΔW
d PV=constants
Solution-1:
In an Isothermal Process
Temperature remains constant ΔT =0
Since Internal energy depends on the temperature
ΔU=0
From first law of Thermodynamics
ΔU=ΔQ-ΔW
Since ΔU=0
ΔQ=ΔW
Also PV=nRT
As T is constant
PV= constant
Question-.2 Two absolute scales A and B have triple points of water defined as 200A and 350A. what is the relation between TA and TB
Solution-2
Given that on absolute scale
Triple point of water on scale A = 200 A
Triple point of water on scale B = 350 B
Also, triple point of water on Kelvin scale = 273.16 K
Now temperature on scale A and on scale B is equivalent to 273.16 K on absolute temperature scale.
Thus, value of one degree on absolute scale A = (273.16/200) K
Or,
Value of temperature TA on absolute scale A = (273.16XTA)/200
Similarly value of temperature TB on absolute scale B = (273.16XTB)/350
Since TA and TB represent the same temperature
273.16×TA/200 = 273.16×TB/350
Or, TA = 200TB/350 = 4TB/ 7
Question 3:A gas is contained in a cylinder with a moveable piston on which a heavy block is placed. Suppose the region outside the chamber is evacuated and the total mass of the block and the movable piston is 102 kg. When 2140 J of heat flows into the gas, the internal energy of the gas increases by 1580 J. What is the distance s through which the piston rises?
Solution:3
Total heat supplied =Workdone + Change in internal energy
So work done=2140-1580=560 J
Let s be the distance moved then
the workdone is given by =Fs
Fs=560
s=560/F
=560/102*10
s=.54 m
Question-4: At 27°C,two moles of an ideal monoatomic gas occupy a volume V.The gas is adiabatically expanded to a volume 2V.
Calculate the ratio of final pressure to the intial pressure
Calculate the final temperature
Change in internal energy
Calculate the molar specific heat capacity of the process
Solution-4
Given
n=2 T=27°C=300 K ,V1=V,V2=2V
Now PVy=constant
P1V1y=P2V2y
P2/P2=(V1/V2)y
=.55/3
ALso
T1V1y-1=T2V2y-1
or T2=300/25/3=189K
Change in internal energy=nCvΔT
For monoatomic gas Cv=3R/2
Substituting all the values
Change in internal energy==-2764J
As in adiabatic process ΔQ=0,molar specific heat capacity=0
Question-5
An ideal gas heat engine operates in Carnot cycle between 227°C and 127°C.It absorbs 6*102 cal of heat at the higher temperature.Calculate the amount of heat supplied to the engine from the source in each cycle
Solutions-5:
T1=227°C =500K
T2=127°C =400K
Efficiency of the carnot cycle is given by
=1-(T2/T1)=1/5
Now also efficency =Heat supplied from source/Heat absorbed at high temperature
so Heat supplied from source=6*102*(1/5)==1.2*102cal

No comments:
Post a Comment